This is pretty good stuff and I have written about FDA Finally Approves AliveCor Iphone Enabled Heart Monitor, Now and the Cat and Dogs Have to Share the Technology With Us
While Waiting for FDA Approval for an IPhone ECG Company Launches Popular Veterinary Version–Cats and Dogs Get Heart Attacks Diagnosed
Now that the algorithm has been approved by the FDA, it should be available within a month or two and the company hopes to develop even more algorithms in time. The AliveCor Heart Monitor records, stores and transfers ECG rhythms and detects presence of atrial fibrillation. I think the device is great for a doctor to carry around with them if they desire.
A physician can integrate the reports with an EMR as well. So far the only one I see listed is Practice Fusion and it does cost $15 a month for the service but you do get the device free which is normally $199 alone. This is the type of mHealth apps and devices I like to see and again this is way more than just a simple consumer app. BD
AliveCor, Inc. announced today that the U.S. Food and Drug Administration (FDA) has granted the company clearance for its algorithm to detect atrial fibrillation (AFib), the most common form of cardiac arrhythmia. AliveCor's automated analysis
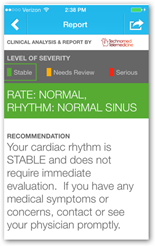 process (algorithm) instantly detects if patients are experiencing AFib through real-time electrocardiogram (ECG) recordings taken on the mobile phone based AliveCor® Heart Monitor, so physicians can intervene before potentially life-threatening conditions, like strokes, occur. Through AliveCor's ECG analysis service, patients can confirm their results with a U.S. board-certified cardiologist or a personal physician.
process (algorithm) instantly detects if patients are experiencing AFib through real-time electrocardiogram (ECG) recordings taken on the mobile phone based AliveCor® Heart Monitor, so physicians can intervene before potentially life-threatening conditions, like strokes, occur. Through AliveCor's ECG analysis service, patients can confirm their results with a U.S. board-certified cardiologist or a personal physician. "The ability to automatically detect serious heart arrhythmia using mobile technology has the potential to save lives, reduce healthcare costs and allow patients and their caregivers to make informed decisions about cardiac care," said Euan Thomson, president and chief executive officer of AliveCor. "Having achieved clearance, we will work to incorporate the algorithm in our app and plan to make this available to customers during September."
The AliveCor Heart Monitor is intended to record, store and transfer electrocardiogram (ECG) rhythms. The AliveCor Heart Monitor also displays ECG rhythms and detects the presence of atrial fibrillation (when prescribed or used under the care of a physician). The AliveCor Heart Monitor is intended for use by healthcare professionals, patients with known or suspected heart conditions and health conscious individuals. The AliveCor Heart Monitor is compatible with all iPhone models and most Android mobile devices. Users will continue to have the ability to access their data confidentially anytime, anywhere.

